New model of molecular reaction of vinylidene cyclopropane discovered by Shanghai Organic
Vinylidenecyclopropanes (VDCPs) are high-tensile molecules that are relatively stable at room temperature. This type of compound molecule consists of two parts: a three-membered ring structure and a propadiene structure (Figure 1 ). Because one of the terminal carbons in the accumulated double bond belongs to a carbon in cyclopropane, such a structure allows vinylidene cyclopropane compounds to include part of the properties of cyclopropane compounds, and the presence of extra-ring double bonds So that its three-membered ring is subjected to greater spatial tension than the ordinary three-membered ring. Since cyclopropane has similar chemical structure to the carbon-carbon double bond of olefin, it is easy to chemically react with various chemical reagents (electrophilic reagents, nucleophilic reagents and free radical reaction reagents, etc.) to generate many different types of compounds , Especially when different substituents are attached to cyclopropane, the diversity of its products is increased. At the same time, the compound has multiple reaction sites due to the presence of propylene groups outside the ring.
Shi Min's research group of the State Key Laboratory of Metal-Organic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences has long been engaged in the study of the reaction of high-tensile molecules, and systematically studied the ring-opening series reaction of vinylidene cyclopropene compounds. It was first discovered in 2005 that it could be ring-opened and rearranged into two novel compounds under the action of metal Lewis acid catalyst (Figure 2). The work was published in J. Am. Chem. Soc. 2005, 127, 14552-14553 and J. Org. Chem. 2007, 72, 509-516, etc.
In 2010 Chem. Rev. 2010, 110, 5883-5913 (shown in Figure 3), a new reaction model of vinylidene cyclopropane tension molecules was summarized. Under the action of Lewis acid (LA) and protonic acid (BA), the carbon-carbon bonds at the proximal or distal ends can be broken to carry out a tandem reaction, and the carbon-carbon bonds at the distal end are mainly broken. Under the action of Brønsted base (BB) such as LDA or nBuLi, it can generate various types of carbon anions and various electrophilic reagents for selective series reaction.
In 2011, it was further discovered that vinylidene cyclopropane and alkyne can be catalyzed by monovalent metal Rh in the intramolecular series cyclization reaction shown in the figure below to obtain polycyclic and macrocyclic compounds (Figure 4). The work was published in Angew. Chem. Int. Ed. 2011, 12027-12031.
The research work was supported by the National Natural Science Foundation of China, the Ministry of Science and Technology and the Shanghai Municipal Science and Technology Commission.
Figure 1. Structure of vinylidene cyclopropane
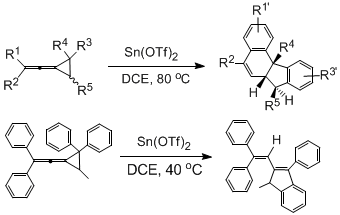
Figure 2. Sn (OTf) 2 catalyzed ring-opening series reaction of vinylidene cyclopropene compounds
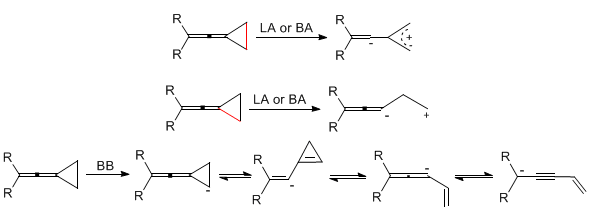
Figure 3. New reaction mode of vinylidene cyclopropane tension molecules
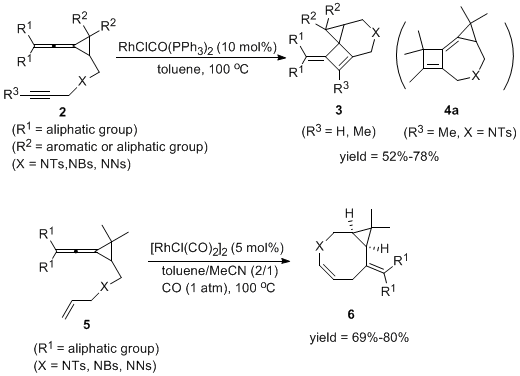
Figure 4. Intramolecular tandem cyclization catalyzed by Rh (I)
Refrigerator Organizer Bins ,Fridge Organization Bins,Refrigerator Storage Bins,Fridge Storage Bins
NINGBO HOZ TRADING CO.,LTD , https://www.hoz-kitchenware.com